Why Is Water Less Dense as a Solid
Why solid water is less dense than liquid water. The density of ice is 09168gcm-3 at 0 C The density of liquid water is 09999gcm-3 at 0 C.
Rather than the molecules packing more tightly together and creating a heavier.

. The liquid phase is less dense than the solid phase but more dense than the gas phase and the gas phase is less dense than either the solid or. Begingroup Possible duplicate of Density of Solid States of Compounds endgroup Floris. As ice water molecules form four hydrogen bonds that lock them into a rigid crystalline structure.
TextWhy is ICE less dense than liquid water Well because God wanted it that way. Hydrogen bonding makes ice less dense than liquid water. The water molecules are pushed farther apart compared to liquid water.
13 Why does water expand when it goes from a liquid to a solid group of answer choices. 12 What occurs when water freezes quizlet. This is why water expands as it freezes and is less dense than the surrounding liquid water.
Why Is Ice Lighter Than WaterThis is due to ices density being less than liquid waters density. Ice is less dense than water because the orientation of hydrogen bonds causes molecules to push farther apart which lowers the density. 16 Why does water freeze in the freezer.
Solid water or ice is less dense than liquid waterIce is less dense than water because the orientation of hydrogen bonds causes molecules to. That the solid phase is less dense than the liquid phase is an highly unusual phenomenon. Waters lower density in its solid form is due to the way hydrogen bonds are oriented as it freezes.
14 When water freezes it is less dense than liquid water Why quizlet. Water is one of the few substances on Earth that is less dense as a solid than a liquid. When water freezes water molecules form a crystalline structure maintained by hydrogen bonding.
However for most substances the density of the solid state is usually larger than. So this right over here is less dense. This is why ice floats.
15 Can water freeze without expanding. Solid water or ice is less dense than liquid water. If it were the other way round as is usually observed for phase transitions ice-bergs would sink rather than float and this planets oceans would probably be ice-bound.
Density of ice is less than the density of water. And for a fact it is unusual that the density of liquid water is GREATER than that of its ICE solid phase under equivalent conditions. The density of water is quite unique as compared to other liquid substances.
Water is unusual in that its maximum density occurs as a liquid rather than as a solid. Because I was told at school that solid form of any given element is more dense than their liquid form. Apr 21 2017 at 1633.
The density of water is maximum at 4 as the temperature decreases the density also decreases. Water molecules are held further apart in ice than they are in liquid water because each water molecule forms four hydrogen bonds to other water molecules making a lattice shape. Therefore lakes freeze top down in the winter.
Why is ice less dense that water. Answer 1 of 9. But I was drinking a glass of water with ice in it and I realized that the ice was floating.
Also as the temperature increases the density of the water starts decreasing. This is more dense more dense. And this is less dense.
Ice is less dense as a solid than as a liquid ice floats Liquid water has hydrogen bonds that are constantly being broken and reformed. 11 When water freezes its volume increases or decreases. The water molecules are pushed farther apart compared to liquid water.
The additional space created reduces the density of the water as it freezes making ice less dense than water. In water ice has a definite structure where each oxygen sits at the approximate centre of a tetrahedron with two bonds to hydrogen and two hydrogen bonds to the hydrogens of other water molecules. The mass of material does not change but the volume or space that it occupies either increases or decreases with temperature.
When water freezes water molecules form a crystalline structure maintained by hydrogen bonding. Given that ice is less dense than water why doesnt it sit completely atop water rather than slightly submerged. With most other liquids solidification when the temperature drops includes the lowering of kinetic energy between molecules allowing them to pack even more tightly than in liquid form and giving the solid a.
Frozen water forms a crystal-like lattice whereby molecules are set at. In liquid water the structure is far more fluid and averages out to be slightly more dense that the more ordered solid. Upon freezing the density of ice decreases by about 9 percent.
When were talking about water when were talking about water when we go to the liquid state when we go from liquid water to solid ice to solid ice we actually get we actually get less dense. For all substances density changes with temperature. In this state the water molecules are actually further apart than when they are in a liquid state.
Waters lower density in its solid form is due to the way hydrogen bonds are oriented as it freezes. Ice floats because it is less dense than water. Why is water less dense as a solid than as liquid.
Why is Water Less Dense as a Solid. Therefore during the cold season a layer of ice will form on the surface of ponds and lakes creating an insulation for the aquatic lives underneath. Water is made up of two hydrogen atoms and one oxygen atom.
This means ice floats on water. It happens that the lattice arrangement allows water molecules to be more spread. Ice is less dense than water because when it freezes the molecules arrange themselves into a highly ordered crystal structure that leaves spaces.
When water is frozen into ice the change in temperature creates excess hydrogen bonds between water molecules that increase the space between the molecules. Density is the mass per unit volume of a material.

Interesting Facts About Water Water Facts Facts Water Molecule

Pin By Eli Solomon On Properties Of Water Surface Tension Molecules Surface

Physical Properties Of Matter Discussion Questions Task Cards Digital Slides Physical Properties Of Matter Properties Of Matter Physical Properties

Because Of Hydrogen Bonding Water Is Less Dense As A Solid Than It Is As A Liquid Consequently Ice Floats Chemistry Outline Biology

Properties Of Water Lesson Plan A Complete Science Lesson Using The 5e Method Of Instruction Water Lessons Science Lessons Chemistry Lesson Plans

Rice Husk Ash Suppliers Conduction Convection Radiation Heat Insulation Rice Hulls

Happy Birthday Science Penguin Science Penguin Matter Science Science

Matter Notes Pre Ap Biology Nursing School Notes Science Notes School Organization Notes
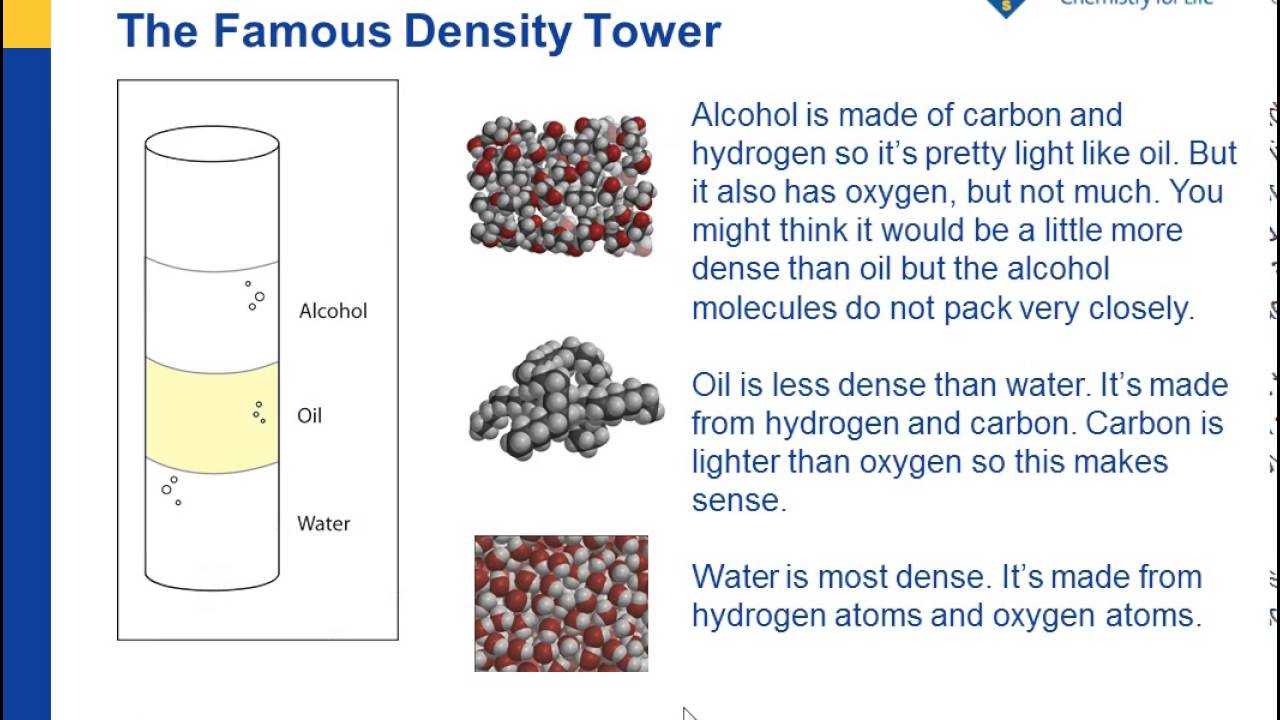
Density Sink And Float For Liquids Chapter 3 Density Middle School Chemistry Middle School Chemistry Teaching Chemistry Chemistry

Water Less Dense As Ice Why Lakes Don T Freeze Solid Winter Math Winter Math Kindergarten Ice Video

Ice Floats Because Solid Water Is Less Dense Than Liquid Water

P13 Density Teaching Science Elementary Science Science Lessons

Surfer S Tension By 4mule8 Best Puns Surfer 3d Printing

Pin On The Pensive Sloth S Pins

Concept Cartoon This Concept Cartoon Titled Added Energy Could Be Used To Assess Students On Their Understanding Of Particle Understanding Particles Energy




